Description
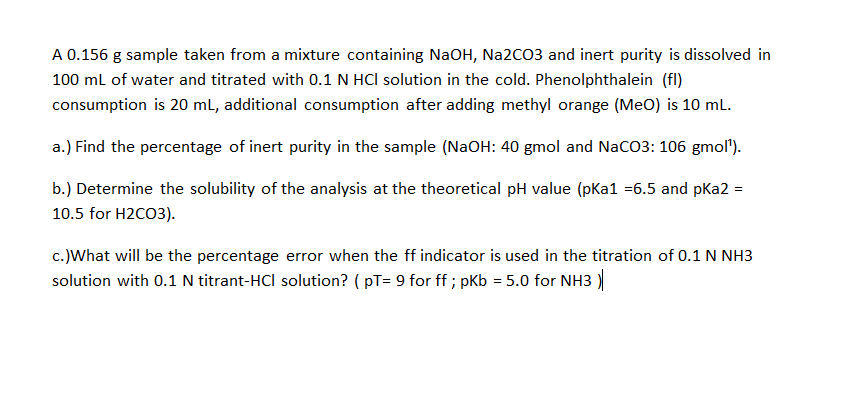
A 0.156𝑔 sample taken from a mixture containing NaOH,𝑁𝑎2𝐶𝑂3 and inert purity is dissolved in
100𝑚𝐿 of water and titrated with 0.1𝑁𝐻𝐶𝑙 solution in the cold. Phenolphthalein (fI)
consumption is 20𝑚𝐿, additional consumption after adding methyl orange (𝑀𝑒𝑂) is 10𝑚𝐿.
a.) Find the percentage of inert purity in the sample ( NaOH:40gmol and NaCO3:106𝑔𝑚𝑜𝑙1 ).
b.) Determine the solubility of the analysis at the theoretical 𝑝𝐻 value ( pKa1=6.5 and pKa2=
10.5 for 𝐻2𝐶𝑂3 ).
c.) What will be the percentage error when the 𝑓𝑓 indicator is used in the titration of 0.1𝑁𝑁𝐻3
solution with 0.1𝑁 titrant- 𝐻𝐶𝑙 solution? ( 𝑝𝑇=9 for 𝑓𝑓;𝑝𝐾𝑏=5.0 for 𝑁𝐻3 )|


Reviews
There are no reviews yet.