Description
A solution in which acetic acid (𝐶) is dissolved in water (𝐴) is extracted using isopropyl ether (𝐶) as a solvent.
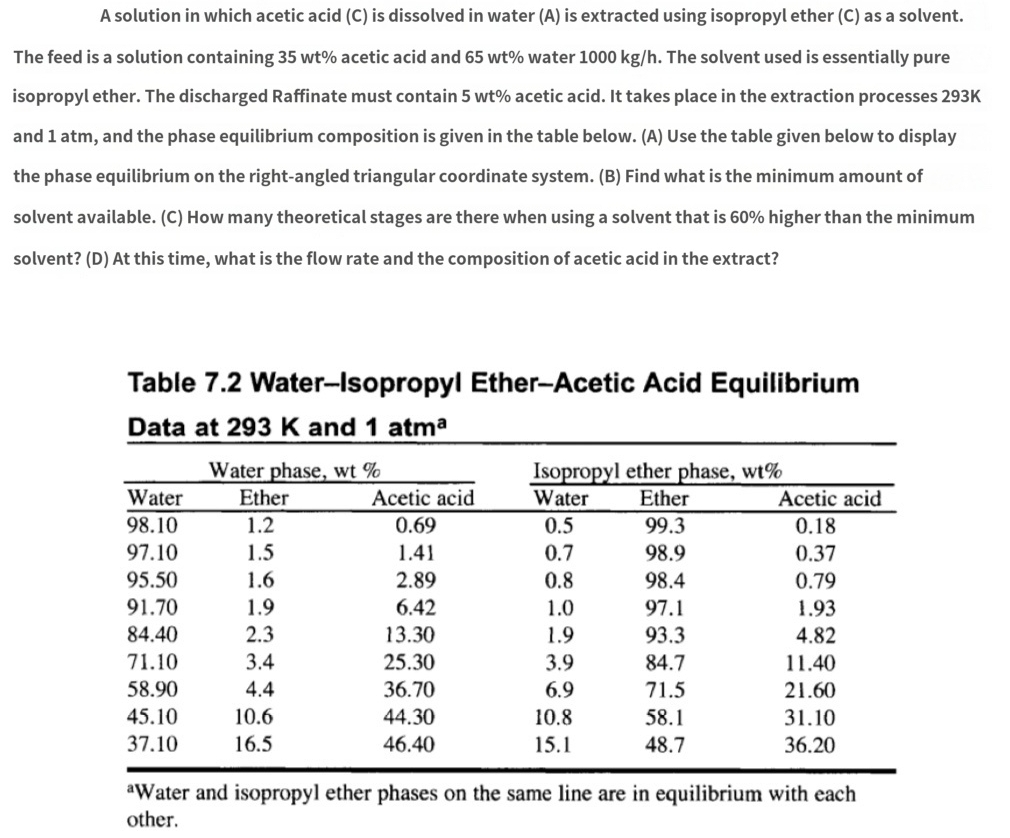
The feed is a solution containing 35𝑤𝑡% acetic acid and 65𝑤𝑡% water 1000𝑘𝑔ℎ. The solvent used is essentially pure isopropyl ether. The discharged Raffinate must contain 5𝑤𝑡% acetic acid. It takes place in the extraction processes 293K and 1atm, and the phase equilibrium composition is given in the table below. (A) Use the table given below to display the phase equilibrium on the right-angled triangular coordinate system. (B) Find what is the minimum amount of solvent available. (C) How many theoretical stages are there when using a solvent that is 60% higher than the minimum solvent? (D) At this time, what is the flow rate and the composition of acetic acid in the extract?
Table 7.2 Water-Isopropyl Ether-Acetic Acid Equilibrium Data at 293𝐾 and 1𝑎𝑡𝑚𝑎
\table[[,Water phase, wt %,,Isopropyl ether phase, wt %


Reviews
There are no reviews yet.